Research
The truth is out there.
Research in the Ohashi Lab (aka Ecological Interactions Lab) covers a wide range of topics, with the common theme that they all involve plant-animal interactions. The Ohashi Lab uses field, indoor experimental, and computational approaches to elucidate how plants have evolved various floral traits and combinations to maximize fitness, mediated through interactions with diverse organisms. We often focus on how flower visitors such as bumble bees and other insects respond to variation in a set of floral traits at different spatial scales, based on foraging economics that has been neglected in the past studies of floral evolution. We have learned from our research that one could effectively predict and test what behavioral responses floral traits have evolved to elicit from animals by incorporating knowledge from behavioral ecology, animal physiology, and animal psychology. To promote such interdisciplinary research in evolutionary ecology, we have also collaborated with researchers from diverse fields, such as behavioral ecologists, animal physiologists, plant taxonomists, chemical ecologists.
Our current collaborators are: James Thomson (Emeritus Professor, University of Toronto, Canada), Tetsukazu Yahara (Emeritus Professor, Kyushu University, Japan), Andreas Jürgens (Professor, Technical University Darmstadt, Germany), Klaus Lunau (Emeritus Professor, Heinrich Heine University Düsseldorf, Germany), Nobumitsu Kawakubo (Professor, Gifu University), Lina Kawaguchi (Research Administrator, Kyoto University), Miki Suzuki (Reserach Associate, Yamanashi University), Daichi Funamoto (PhD Student, Kobe University), Martin Burd (Associate Professor, Monash University, Australia; with many others in international project on the evolution of floral color in angiosperms).

Main interests
The followings are some selected topics in which we are currently interested. As you can tell from the interests of individual members (see 'People' page), a lot more is going on in the lab. Not just flowers and insects, we are also interested in the interactions of a wide range of organisms, such as the relationships between individuals of the same species, between prey and predator, and between human activities and organisms. Those who wish to work with us on slightly different topics are also welcome.
1. Manipulation of pollinator behavior by flowers
Probing many flowers on the same plant could save flight costs for pollinators. But it causes a problem for plants, because it increases self-pollination. Plants can reduce this cost of self-pollination by having complex flowers that require pollinators to take longer for drinking nectar (Ohashi 2001).
The cost of visiting a flower for a pollinator is the sum of travel time to the flower and the time for extracting nectar from the flower. Therefore, when a pollinator has to spend more time for drinking nectar, the difference in travel time for inter-plant and intra-plant movements will become insignificant. In this situation, the pollinator should move between plants frequently to avoid the increasing risk of flower revisitation on the same plant. Which will lead to a reduction of self-pollination for the plants.
Plants are often thought to be static. But as the above example, plants may have evolved floral traits that can manipulate pollinator behavior for their own good. We are particularly interested in exploring the mechanisms of such behavioral manipulation of pollinators by flowers.

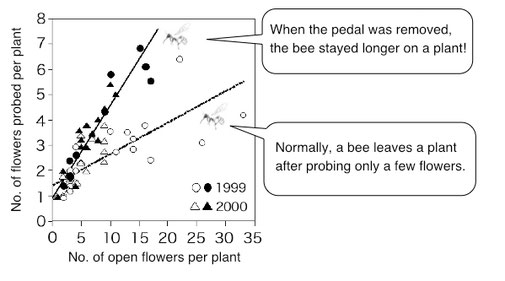
Bombus diversus has a long tongue and can extract nectar from the deep tubular flowers of Salvia nipponica. However, the flower entrance is blocked by a pedal-like structure, and a bee has to push up the pedal with its head before inserting its tongue (above, from "Field Watching: Walking in the Fields of Autumn [Hokurukan]"). In a field experiment where the pedals were removed from the entire population, each bee stayed longer on a plant (modified from Ohashi 2001).
2. Site-faithful foraging by pollinators
Bumble bees are highly efficient foragers for pollen and nectar, and one of the major pollinators in temperate zones. As they gain experience, individual bees learn the locations of their favorite plants and visit them repeatedly along their favorite routes, i.e., traplines (Makino, Ohashi & Sakai 2007, see below).
Trapliners could avoid immediate returns to the same plants until nectar is refilled in flowers. This would benefit plants, too, because pollen could be delivered farther from the source plants (Ohashi & Thomson 2009). At the same time, trapliners may be a vexing problem for plants. For example, if plants reduce the nectar production to save energy, the bees will quickly learn to avoid such "cheater" plants.
In other words, bumble bees are "dependable but demanding customers" for flowers. We are interested in how flowers could effectively use them for pollination.

Bumble bees visiting a natural population of Cirsium purpuratum were individually numbered and their movements from plant to plant were recorded. We found that individual bees often followed fixed foraging routes (Makino, Ohashi & Sakai 2007). In addition, these traplining bees often incorporated plants with larger displays into their routes.
This has also been demonstrated in indoor cage experiments with artificial flowers secreting nectar at a constant rate. As bees gain foraging experience, they remember more rewarding plants and return to them more frequently (Ohashi et al. 2007).
Trapliners do not waste time moving back and forth between neighboring plants. This gives some advantages in competition with other foragers. Computer simulations have shown that these movements of trapliners also have a positive effect on the plant by delivering more pollen to a greater distance (Ohashi & Thomson 2009).
3. Floral color change for a full exploitation of behaviorally diverse pollinators
Floral color has been thought to be an important signal to pollinators. However, some plants dramatically change this signal in the middle of blooming (Ohashi et al. 2015, see below). In most cases, these plants retain old, non-reproductive, and rewardless flowers in an altered color.
The retention of old flowers will increase the size of floral display, thereby luring opportunistic foragers (e.g., hoverflies). However, such false advertisement may not work for more cognitively sophisticated foragers (e.g., bumble bees), because they could develop learned preferences toward individual plants (see 2.). The aversion to displays with old flowers in sophisticated foragers, however, may decrease as plants increase the level of honest signals, i.e., the amount of color change in old flowers. In this way, floral color change allows the plant to exploit behaviorally diverse pollinators.
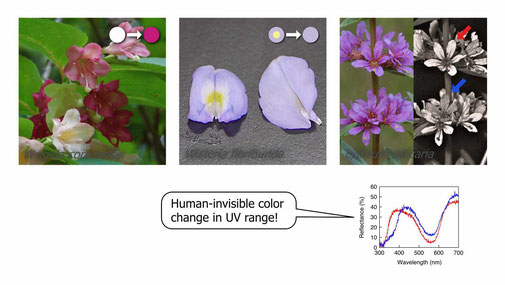
A screening search for color change in 219 angiosperm species has revealed that ~20% of investigated species exhibit pronounced floral color change, and another 1/4 of them do so only in the ultraviolet region, which is invisible to humans (Ohashi et al. 2015). In some species, the whole flower change its color, while in others it occurs only partially. The reason for it has not been determined yet.
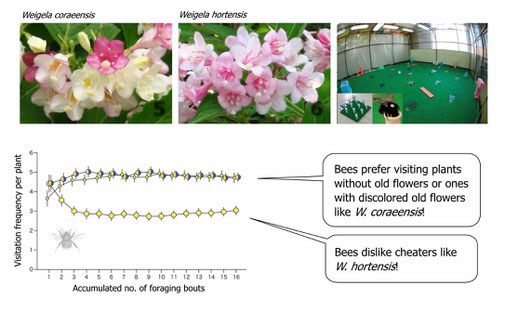
In a botanical garden we compared between a color-changing species (upper left) and its non-color-changing congener (upper middle). The former species attracted more bumble bees (Suzuki & Ohashi 2014). In indoor experiments with bumble bees (upper right), similarly, bees always learned to selectively visit plants without old flowers or color changers, while avoiding ones with non-color-changing old flowers (bottom; modified from Makino & Ohashi 2017).
4. Anthesis timing as the determinant of arrival sequence of diverse pollinators
For flowers that bloom for more than 24 hours, the time they first open does not seem to matter much. However, many flowers open at a certain time of day, and the timing varies across plant species—some open in the morning, while others open in the evening. Why?

Adenophora flowers that open in the evening are primarily pollinated by nocturnal settling moths. Bees and hoverflies also visit the flowers during the daytime, but their pollination efficiency is much lower (Funamoto & Ohashi 2017, see below). Thus, the dusk opening in Adenophora flowers prioritizes visits by nocturnal moths and avoid the waste of pollen, nectar, and stigmatic surface by less efficient diurnal pollinators. We have recently found that a similar adaptation in anthesis timing of Salix caprea that blooms early spring. Salix flowers depend largely on diurnal pollination by bees and wind. But they open in the evening to leave the opportunity for pollination by nocturnal moths that is supposed to be infrequent but more efficient in terms of pollen transfer to stigmas (Ohashi & Jürgens 2021).
It will be interesting to see whether similar explanations apply to the anthesis timing of other species that are visited by a set of diverse pollinators varying in their activity time and pollination efficiency.

The flowers of Adenophora open just after sunset. Although the flowers last for several days, they secrete nectar only at night. The results of bagging experiments showed that pollination is carried out exclusively by nocturnal settling moths (1, 2), while diurnal bees (3) and hoverflies (4) visiting the flowers on the following day do not make much contribution, wasting pollen and nectar (Funamoto & Ohashi 2017). The dusk opening of Adenophora flowers is considered a strategy to prioritize the visits by moths while not ruling out the possibility of pollination by diurnal insects as "insurance" in case moths are infrequent.
There are two possible outcomes:
If the result confirms the hypothesis, then you've made a measurement.
If the result is contrary to the hypothesis, then you've made a discovery.
— Enrico Fermi
Copyright(c)2018 Ohashi Laboratory All Rights Reserved.